Sickle cell disease medication Oxbryta has been linked to severe pain, serious complications, and death in patients. Find out more about the Oxbryta recall and whether you may be eligible to participate in a lawsuit against the manufacturers.
Reviewed by David A. Neiman, Esq.
Image by Pixabay.
Timeline of the Oxbryta Lawsuits
September 26, 2024 — The Food and Drug Administration (FDA) announces Pfizer’s voluntary withdrawal of Oxbryta to the public.
July 29, 2024 — The European Medicines Agency suspends Oxbryta’s marketing privileges in the European Union based on recent studies and requests more research.
2022 — Pfizer acquires Global Blood Therapeutics in a $5 billion deal.
February 2022 — The European Medicines Agency approves Oxbryta.
2021 — The FDA approves Oxbryta for use in patients aged 4 to 11.
2019 — The FDA approves Oxbryta for use in patients 12 and up under its Accelerated Approval Program.
What is sickle cell disease?
Sickle cell disease is a group of red blood cell disorders that affects 30 million people worldwide and an estimated 100,000 people in the US. A lifelong condition, it is diagnosed via a blood test, usually through routine newborn screenings or diagnostic tests in the womb. Sickle cell disease is most common in people of African, Middle Eastern, and South Asian descent.
As a category of genetic blood disorder, sickle cell disease is inherited from one’s parents through genes that contain a “code” for abnormal hemoglobin cells. Hemoglobin is a protein in red blood cells that carries oxygen around the body. In people with sickle cell disease, a genetic mutation affects cells’ ability to do their jobs.
Healthy red blood cells are oval-shaped, flexible, and move easily through the body. However, in people with sickle cell disease, red blood cells are shaped like a crescent or sickle (giving the disease its name). These cells are harder and stickier than healthy cells and do not move or bend as easily, impacting their ability to move through blood vessels. They also break down more quickly than healthy cells, which can lead to a consistent red blood cell shortage in the body.
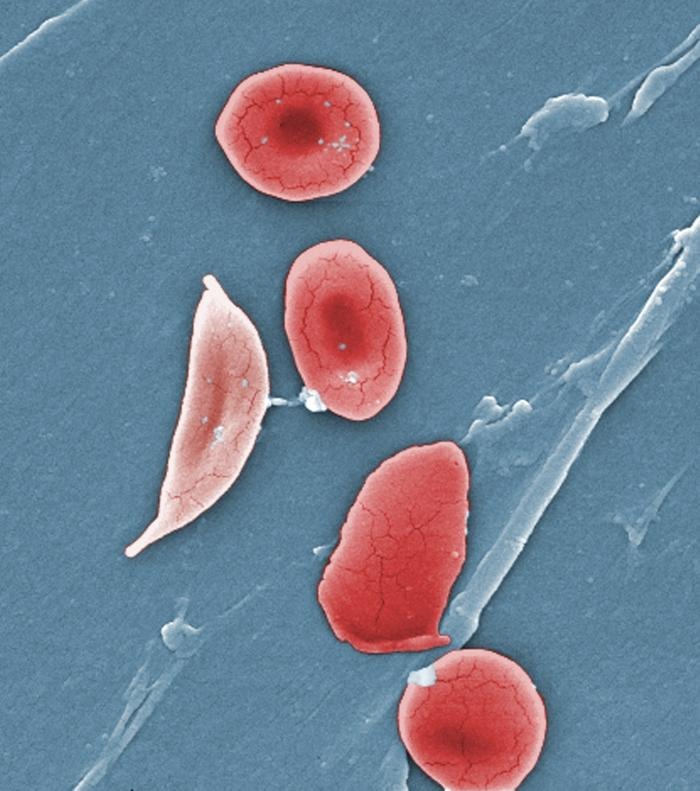
Normal red blood cells and a sickle cell red blood cell (left). Image by Janice Haney Carr in the Public Domain.
Types of sickle cell disease
Genes are pieces of DNA with “instructions” for creating proteins, passed from parent to child. There are two genes that can affect people with sickle cell disease, one inherited from each parent. The type of sickle cell disease depends on the combination of those two genes.
Hemoglobin SS (HbSS)
Individuals with HbSS inherited one gene with hemoglobin S from each parent. This is the most common type of sickle cell disease, occurring in about 65% of individuals affected. HbSS is typically severe, as most or all of the person’s hemoglobin is abnormal, leading to chronic anemia.
Hemoglobin SC (HbSC)
Patients with HbSC inherited hemoglobin S from one parent and hemoglobin C, another abnormal gene type, from the other parent. HbSC makes up about 25% of all sickle cell disease and is typically mild to moderate.
Hemoglobin (HbS) beta thalassemia
Occurring in about 10% of cases, HbS beta thalassemia patients inherited hemoglobin S from one parent and beta thalassemia (another abnormal gene) from the other. These cases are divided into two subtypes:
- HbS beta + (or “Plus”): Occurs in 8% of sickle cell patients; typically less severe.
- HbS beta 0 (or “Zero”): Occurs in 2% of sickle cell patients; typically more severe.
There are a number of other rare subtypes caused by inheriting hemoglobin S alongside another abnormal gene, including hemoglobin SD, hemoglobin SE, and hemoglobin SO.
Sickle cell anemia
The term “sickle cell anemia” is used by healthcare professionals to refer to the types of sickle cell linked with the most severe anemia, usually hemoglobin SS and HbS beta 0. These types of sickle cell disease can lead to the excess breakdown of red blood cells and severe complications as a result.
Sickle cell trait
Some people inherit the sickle cell gene from one parent and a normal gene from the other. These people usually have no symptoms and can pass on the gene to their children. However, there is evidence that carrying the sickle cell trait provides some protection against contracting malaria, which may explain why the gene remains so common in some segments of the global population.

Image by Pixabay
Severe complications and symptoms of sickle cell disease
Sickle cell disease can cause a range of health complications and symptoms from mild to severe. Symptoms of the disease may include:
- Severe, persistent, or chronic pain. Pain is a common symptom in individuals with sickle cell. An acute episode of pain is called a vaso-occlusive crisis (VOC), vaso-occlusive episode (VOE), or a sickle cell crisis. These episodes usually occur in the chest, back, legs, and arms. They can be mild to severe, start suddenly, and last for any length of time.
- Anemia. Caused by a shortage of red blood cells, anemia can lead to fatigue, weakness, irritability, lightheadedness, and other symptoms.
- Jaundice, or yellowing of the skin and the whites of the eyes.
- Swelling or inflammation of joints, hands, and feet.
Other complications of sickle cell disease can include:
- Acute chest syndrome, in which sickled cells block blood and oxygen from the lungs
- Blood clots
- Organ damage or failure, especially in the heart, lungs, and kidneys
- Infections
- Chronic pain
- Stroke
- Eye problems
Can you treat sickle cell disease?
Sickle cell disease is a lifelong condition. A bone marrow transplant can cure the disease, but it requires a donor who’s a genetic match, such as a sibling or other relative. Most people with sickle cell do not have a compatible donor, and for those that do, the procedure can be risky, expensive, and lead to further complications.
There are several treatments for sickle cell disease management, including:
- Medications (such as crizanlizumab, hydroxyurea, and L-glutamine)
- Regular blood transfusions
- Blood and marrow transplants
- Gene therapy (currently in development, this treatment involves working directly with the mutated gene)
Although individuals with sickle cell disease have a reduced life expectancy, treatment continues to improve, and they can still leave a full and happy life. There is a significant need for more and better treatments for sickle cell patients worldwide.
The development of Oxbryta
Oxbryta was developed by Global Blood Therapeutics, a pharmaceutical company focusing on sickle cell treatments that was later acquired by Pfizer. In two early studies, voxelotor—the active ingredient in Oxbryta—demonstrated links to a significant increase in hemoglobin for those with sickle cell.
In a study published in Blood in January 2019, voxelotor seemed to limit the sickling of cells and help cells carry more oxygen in patients taking the medication for more than 28 days. Researchers reported a substantial increase in hemoglobin for many patients, a reduction in the number of sickled cells, and no adverse events over the course of the study.
Another study, published in the New England Journal of Medicine in June 2019 and sponsored by Global Blood Therapeutics, found that voxelotor provided a “significant, sustained increase in hemoglobin level and reduced the incidence of worsening anemia and hemolysis in persons with sickle cell disease.” In other words, the study showed that voxelotor helped increase the amount of hemoglobin in patients’ blood, prevented their anemia from getting worse, and prevented their red blood cells from breaking down. Although four people died during the study, their deaths were found to be unrelated.

Image by Pixabay.
How does Oxbryta treat sickle cell disease?
Initial Oxbryta trials suggested that the voxelotor prevented sickling, allowing cells to remain oval-shaped and flexible. By helping red blood cells maintain their normal shape and flexibility, the medication could reduce the hemolysis, or breakdown, of red blood cells. This increased their lifespan and decreased the risk of anemia. Oxbryta also appeared to improve the ability of hemoglobin to hold onto oxygen and prevent hemoglobin from forming chains that could then block blood vessels.
According to Global Blood Therapeutics advertisements, Oxbryta promised to treat sickle cell disease “at the source” by preventing sickling and the breakdown of red blood cells. Its listed side effects included headache, diarrhea, stomach pain, nausea, tiredness, rash, and fever.
FDA approval of Oxbryta
Oxbryta was approved by the US Food and Drug Administration (FDA) in 2019 under the Accelerated Approval Program. This program provides a system for some drugs to receive early approval based on promising clinical studies if they treat serious conditions and fill an unmet need. These drugs are typically required to undergo more studies after approval to verify the drug’s clinical benefits.
From its approval in 2019, Oxbryta was marketed as a safe, effective treatment for sickle cell disease for those 12 and older. In 2021, the FDA approved the medication for patients 4 to 11 years old. Oxbryta was also approved for use in the EU through the European Medicines Agency on an accelerated basis in 2022.

Image by Pixabay.
Post-approval studies reveal serious health risks
Although initial tests indicated that Oxbryta was a promising treatment for sickle cell patients, studies conducted after the drug was approved demonstrated that vaso-occlusive crises (VOCs) and deaths were higher in people taking the drug than in the control group.
One study was conducted on 236 children aged 2 to 15 with sickle cell disease at high risk of stroke, testing cerebral arterial blood flow. During the research period, there were eight deaths reported in patients taking Oxbryta and only two deaths in the placebo group.
A second study of 88 patients was designed to assess the effect of the drug on leg ulcers in patients 12 and older. Eight deaths occurred in the open-label portion of the study.
In both studies, patients taking Oxbryta reported higher rates of vaso-occlusive crises and pain. As a result of these findings, treatment was paused for patients in both studies.
Pfizer pulls Oxbryta from the market in voluntary recall
In September 2024, the FDA informed the public that Pfizer was voluntarily withdrawing Oxbryta from the market, ceasing distribution, and pausing all further clinical trials. The new clinical data, according to the manufacturer and the FDA, indicated an increased risk profile. The FDA wrote, “The benefits do not outweigh the risks for the sickle cell patient population.”
After the Oxbryta recall, healthcare providers were instructed to no longer prescribe Oxbryta and work with their patients currently on the drug to find another treatment plan.

Image by Pixabay.
Sickle cell patients bring Oxbryta lawsuits
Several lawsuits have been filed in the state of California in both state and federal court. The lawsuits list Pfizer and Global Blood Therapeutics as the primary defendants for their role in manufacturing and marketing the sickle cell disease drug.
Plaintiffs allege that the manufacturers did not adequately warn them of the risks of taking Oxbryta. They claim that their use of Oxbryta led to increased vaso-occlusive crises and strokes, as well as significant emotional and physical pain.
The charges include design defect, failure to warn, negligence, breach of warranties, false advertising, and more. We anticipate that additional Oxbryta lawsuits will be filed as patients learn of the recall and the risks associated with the medication.
About the defendants
Pfizer, Inc. is the primary defendant in the Oxbryta lawsuits so far. A multinational pharmaceutical company, it acquired Global Blood Therapeutics in 2022 for $5 billion and continued to manufacture and market Oxbryta until the voluntary recall in 2024.
Global Blood Therapeutics has also been named as a defendant in Oxbryta lawsuits. Acquired by Pfizer in 2022, it was a pharmaceutical company dedicated to researching and developing sickle cell disease treatments. The company is responsible for Oxbryta’s research, development, and approval in the US and other countries.

Image by Pixabay
The dedicated team at Wallace Miller
With more than 100 years of cumulative experience, our team of attorneys is dedicated to helping those who have been harmed by the actions of businesses and corporations through no fault of their own. You trust that the products approved by pharmaceutical companies are safe to use, and when those products cause harm instead, you deserve justice.
Reach out to our office at (312) 261-6193 or fill out the online case evaluation for a free and confidential consultation with a member of our team. We’ll discuss your case and help you determine if litigation is the best path forward.

Left to right: Nicholas P. Kelly, Edward A. Wallace, Molly Condon Wells, Mark R. Miller, Jessica Wieczorkiewicz, Timothy E. Jackson. Not pictured: Alexandrea M. Messner, Julia Ozello, Kristina J. Anderson, Kathleen B McGivney, David A. Neiman.