
Over the last several years, chronic use of the prescription medication Elmiron has been repeatedly linked to vision problems and eye injury. More than 1,800 plaintiffs have brought Elmiron vision damage lawsuits to court since January 2020, and as of late last year, these eye injury cases have been consolidated in two jurisdictions: one in New Jersey at the federal level (MDL 2973) and one in Pennsylvania at the state level (Mass Tort Program in the Philadelphia Court of Common Pleas). The Elmiron lawsuits claim that the medication caused vision problems including distorted vision, difficulty reading, and vision loss, and that the manufacturers of the drug, Janssen Pharmaceuticals, should have warned doctors and consumers of the possible risks.
Wallace Miller is representing individuals in the Elmiron lawsuits who have developed vision issues as a result of the bladder pain medication. If you believe you have been harmed by taking this medication, reach out to our attorneys who specialize in Elmiron cases at 312-261-6193 or fill out our online questionnaire for an assessment of your case in minutes.
This content was reviewed by Timothy E. Jackson.
Elmiron lawsuit timeline & updates
August 2023: The Wallace Miller law firm and others representing the plaintiffs in the Elmiron vision loss litigation are continuing to make good progress. The Pennsylvania State Court has recently selected the first set of bellwether cases, and of the 10 cases, our firm has been tapped to work on four of them. We are actively conducting depositions of the bellwether plaintiffs and their doctors and preparing their cases for trial. The initial bellwethers are set to occur in spring of 2024.
We know that the speed of the mass tort litigation process can be frustrating. Wallace Miller’s Elmiron lawyers continue to push your litigation forward as quickly and effectively as possible. Through the upcoming bellwether cases, we are one step closer to a potential resolution.
August 2023: Bellwether trials for 10 cases have been identified by the Pennsylvania State Court. Trials are set to begin in spring of 2024.
April 2023: Judge Abbe Fletman of the Philadelphia Court of Common Pleas continues to oversee the Philadelphia State Court Elmiron mass tort program. Wallace Miller attorneys are actively working cases up towards trial, the first of which is scheduled to begin in March 2024. There are now more than 1,880 Elmiron cases included in the Elmiron multidistrict litigation (MDL).
January 2023: After a slow end of 2022, the number of Elmiron MDL cases has passed 1,800.
October 2022: Elmiron MDL Judge Martinotti has postponed the first bellwether trial, initially planned to take place in January 2023.
September 2022: The Philadelphia County Court of Common Pleas in Pennsylvania granted the Elmiron plaintiffs’ petition to coordinate Elmiron vision loss cases in Pennsylvania as a mass tort program in Philadelphia. This order allows all currently filed Elmiron lawsuits to be transferred to the Complex Litigation Center to be coordinated under Master Docket number 220200209. Our Elmiron attorneys are proud to represent plaintiffs in this litigation.
September 2, 2022: More than 1,700 Elmiron cases are pending as part of the Elmiron MDL in New Jersey. The Elmiron MDL judge is currently scheduling bellwether trials to begin in January 2023.
June 15, 2022: The Elmiron MDL continues to grow with more than 1,300 Elmiron lawsuits filed in New Jersey. Elmiron eye damage lawsuits are also coming through the Pennsylvania State Court via Philadelphia Court Common Pleas.
January 2022: There are now more than 600 Elmiron cases in the New Jersey MDL.
February 2021: A Canadian woman files an Elmiron class action lawsuit alleging the drug led to vision loss.
December 2020: The Judicial Panel on Multidistrict Litigation centralizes all current Elmiron lawsuits into an MDL assigned to the US District Court for the District of New Jersey. District Judge Brian Martinotti will oversee the Elmiron lawsuits.
June 16, 2020: The Food & Drug Administration issues a warning of retinal pigmentary changes caused by long-term use of Elmiron. Janssen Pharmaceuticals adds an extensive new warning label to their US packaging.
January 2020: The first Elmiron lawsuit is filed against Janssen Pharmaceuticals, the manufacturer of chronic bladder pain drug Elmiron, alleging that the drug caused severe eye injury and vision impairment and that the manufacturer should have been aware of the risk. In addition, the Elmiron lawsuit states that Janssen Pharmaceuticals withheld adverse event reports from the public and the Food & Drug Administration (FDA).
2019: The manufacturer of Elmiron, Janssen Pharmaceuticals, updates the medication’s warning label in Canada to notify consumers about potential vision damage.
October 2018: Emory Eye Center researchers publish a letter to the Journal of Urology detailing troubling correlations between Elmiron use and serious eye injury and vision loss.
What is the prescription drug Elmiron?
Elmiron is the brand name for pentosan polysulfate sodium, a blood thinning drug that was developed to treat bladder pain associated with a condition called interstitial cystitis (IC). Manufactured by Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, Elmiron is the primary medical treatment for the condition and is the only prescription oral medication approved for use to treat interstitial cystitis in the US. It has been widely prescribed for over 25 years, with many Elmiron plaintiffs reporting use for over 15 years.

The label for Elmiron (pentosan polysulfate sodium). Image courtesy of the Food & Drug Administration.
Bladder pain syndrome and interstitial cystitis
Interstitial cystitis (IC) impacts millions of Americans every year, the majority of whom are women. The condition is part of a category of diseases called painful bladder syndrome and affects the signals your bladder sends to your brain to indicate it’s time to urinate. IC can cause chronic pain that ranges from mild to severe, as well as bladder pressure and increased urinary frequency.
The spectrum of conditions classified under bladder pain syndrome is very poorly understood and the exact cause of IC isn’t known. It may be due to a combination of factors including heredity, autoimmune reactions, infection, or allergy. Some people with IC also have a defect in the lining of the bladder which protects the bladder wall from toxins in urine.
According to the manufacturers, Elmiron prevents the irritation of the bladder wall common in interstitial cystitis by restoring the mucus layer on the inside of the bladder. In other words, it helps improve a “leaky” bladder lining. This prevents substances in the urine from irritating the organ.

The bladder and nearby organs in the female urinary tract. Image courtesy of the National Cancer Institute.
The most common symptoms of interstitial cystitis include:
- Chronic pain in the bladder and/or pelvis
- Frequent and urgent need to urinate
- Urination, often in small amounts, throughout the day and night
- Pain during sex
There is no cure for IC and the condition can have a long-lasting impact on patients’ quality of life. Elmiron is the only medical treatment approved to treat interstitial cystitis in the US.
Side effects experienced by Elmiron patients
Before 2018, the manufacturer of Elmiron claimed that side effects of the medication were usually mild and could include hair loss, diarrhea, nausea, blood in stool, headache, rash, upset stomach, abnormal liver function tests, dizziness, and bruising.
A growing body of research suggests that significant risk of vision impairment may also be a result of long-term use of Elmiron. This potential side effect was never disclosed by Janssen Pharmaceuticals.
The history of Elmiron
The prescription drug Elmiron was initially researched and released by Baker Norton Pharmaceuticals as an anticoagulant, or blood thinner. Baker Norton was later bought by Teva Branded Pharmaceutical Products R&D, which licenses Elmiron to Janssen Pharmaceuticals.
In 1985, Elmiron was granted Orphan Drug designation by the FDA. This designation applies to drugs that are either:
- intended to treat a disease or condition that affects fewer than 200,000 people in the US, or
- intended to treat a disease or condition that affects more than 200,000 people in the US, but the costs of research and development cannot realistically be made up by drug sales.
Products under the Orphan Drug designation receive tax credits of 50% off the cost of clinical drug testing, awarded upon approval. They are also eligible for 7 years of market exclusivity, which means that for seven years after development, a brand-name drug (such as Elmiron) will be protected from competition by generic drug versions.
As an Orphan Drug, Elmiron was also entitled to waive the application fee for the new drug application (NDA) and biologics license application (BLA), a value of about $2.2 million.
The manufacturers filed the first NDA for approval in 1991. In 1993, the FDA issued its first non-approval letter due to issues with the clinical trial results and analyses, and requested new trials be conducted. In the non-approval letter, the FDA wrote:
“The application as submitted lacks substantial evidence consisting of adequate and well-controlled investigations […] that the drug product will have the effect it purports or is represented to have.”
Rather than conducting additional trials, the manufacturer re-analyzed the data from the studies previously submitted and sent back the amended NDA. In 1994, the FDA issued another non-approval letter and once again requested that the sponsor conduct another clinical trial.
Again, the manufacturer declined to conduct another trial. Instead, representatives met with the FDA and proposed conducting an analysis of cases from Baker Norton’s Compassionate Use database. Despite what a reviewer determined was insufficient evidence of the drug’s efficacy, as well as an apparent lack of clinical safety concerns, the FDA issued the NDA approval in 1996. This approval was based on only two studies and had raised repeated red flags throughout the review process.
Does Elmiron help with painful bladder syndrome?
Although Elmiron is the only drug on the US market approved to treat interstitial cystitis, repeated studies have questioned its efficacy. In 2003, a clinical trial published in The Journal of Urology examined the effects of bladder medications Elmiron and hydroxyzine and found that “neither provided benefit for the majority of patients with IC.” In 2014, a much larger, multicenter trial of patients prescribed Elmiron “revealed no treatment effect vs placebo for pentosan polysulfate sodium at the currently established dose or at a third of the daily dose.”
In other words, after dubious initial clinical trials and FDA approval, there has been no conclusive evidence that the drug helps Elmiron users with IC.
Elmiron linked to pigmentary maculopathy
Research since 2018 has indicated that Elmiron use is toxic to the retina, the light-sensing tissue at the back of the eye that allows us to see. Specifically, Elmiron usage has been linked to a new condition called pigmentary maculopathy.
In patients with pigmentary maculopathy, damage to the retina causes the gradual loss of central vision. Specifically, the disease damages the macula: the central part of the retina that is responsible for clear, crisp sight in the center of one’s field of vision. As the vision loss progresses, the pigment in the eye changes color, giving the disease its name.
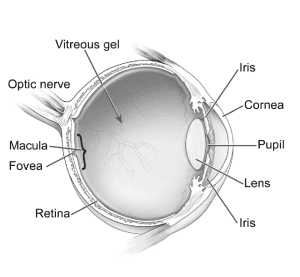
A diagram of the human eye. Photograph by Pixabay.
Patients often have trouble reading, seeing fine details, adjusting to low lighting, and recognizing faces and colors. There is no cure for the permanent vision loss that can occur as a result of pigmentary maculopathy, and the vision damage is often irreversible.
Similar eye diseases are usually related to aging, an underlying disease like diabetes, or genetics. However, pigmentary maculopathy appears unrelated to any of these factors and instead frequently appears in patients who have used Elmiron.
What is pentosan polysulfate sodium?
The primary ingredient in Elmiron, pentosan polysulfate sodium (PPS), is a macro-molecule that prevents irritation of the bladder walls. PPS is derived from a compound called xylan, which is extracted from the bark of beech trees.
There is a growing body of scientific evidence showing that long-term PPS use can cause pigmentary maculopathy and vision loss. Specifically, researchers have noted that pigmented deposits appear on the retina of Elmiron patients who used the drug over many years.
FDA requires Janssen Pharmaceuticals to add a new warning to Elmiron packaging
In June 2020, the FDA approved safety-related information about retinal pigmentary changes (also known as pigmentary maculopathy) to be added to the Warnings and Precautions as well as the Adverse Reactions sections of the Elmiron approved drug label. The new label reads:
“Pigmentary changes in the retina, reported in the literature as pigmentary maculopathy, have been identified with long-term use of ELMIRON®. Although most of these cases occurred after 3 years of use or longer, cases have been seen with a shorter duration of use. While the etiology is unclear, cumulative dose appears to be a risk factor.”
The label cites symptoms including “difficulty reading, slow adjustment to low or reduced light environments, and blurred vision.” In addition, it recommends retinal examinations before, during, and after using Elmiron and notes:
“If pigmentary changes in the retina develop, then risks and benefits of continuing treatment should be re-evaluated, since these changes may be irreversible. Follow-up retinal examinations should be continued given that retinal and vision changes may progress even after cessation of treatment.”
Research linking Elmiron to eye damage
Since the Emory Eye Center’s initial warning of potentially permanent vision damage, a number of studies have corroborated and elaborated on their findings. All of the studies below found symmetric pigmentary changes in the retina linked to vision loss, a classic sign of the new disease. Studies also found that the eye injury increases with the amount of Elmiron taken and is more pronounced in patients with chronic exposure. It is unclear if the development of vision issues is halted when Elmiron intake is stopped.
2019 – Emory Eye Center report
In 2018, Dr. Nieraj Jain at the Emory Eye Center noticed that a number of patients with other eye conditions had been taking the drug Elmiron for a median of 15.5 years. In a letter to the editor of the Journal of Urology, Jain and colleagues wrote, “We wish to alert readers to a concerning new observation of vision threatening retinal changes associated with long-term exposure to PPS. […] After extensive investigations, which included molecular testing for hereditary retinal disease, we found these cases to resemble no other retinal disease.” Experts in interstitial cystitis (IC) responded that the implications that the treatment is causing vision loss are “frightening.”
Based on his research, Dr. Jain observed and described a new eye disease, which he named pigmentary maculopathy due to the fact that the pigment within the retina changes color.
Dr. Jain discovered that the chemical in Elmiron, pentosan polysulfate sodium, accumulates in the cells of the retina in patients who have taken the drug over a long period of time. This accumulation damages the retina and causes significant vision impairment and eye injury. He recommended additional investigation into the new disease.
Notably, the researchers also uncovered that in official clinical trials of 2,499 patients by the manufacturers of Elmiron, both vision problems and eye-related adverse events were reported. Neither of these issues were disclosed on the product warning label and the manufacturer conducted no further tests into potential vision loss.
2019 – Second Emory Eye Center case study
Emory followed this report with a second case study of 10 patients, published in 2019 in the Journal of Urology. The researchers, including Dr. Jain, found structural changes leading to vision impairment in the retinas of these patients, all of whom had taken PPS and experienced macular disease. The study states:
“We describe a potentially avoidable retinal degeneration phenomenon associated with chronic PPS exposure. Structural changes occur at the level of the retinal pigment epithelium, manifesting as characteristic pigmentary changes.
“While it remains unclear whether drug cessation will alter the course of disease, we encourage affected patients to discontinue use, and patients with suggestive visual symptoms to undergo a comprehensive ophthalmic examination with OCT and FAF imaging.”
As noted above, affected patients were encouraged to discontinue use of Elmiron and undergo further testing to determine whether their vision had been affected.
October 2019 – American Academy of Ophthalmology report on Kaiser Permanente Study
After identifying one woman with vision impairment who had been misdiagnosed with retinal dystrophy while using Elmiron, ophthalmologists at Kaiser Permanente in Northern California began a review of their full database of more than 4.3 million patients. As described in a report from the American Academy of Ophthalmology, researchers Robin A. Vora, Amar P. Patel, and Ronald Melles found 140 potentially impacted patients. These patients had all used Elmiron and had taken an average of 5,000 pills over the course of 15 years.
Of the 91 patients who agreed to come back into the clinic for further screening, 22 showed definite signs of drug toxicity, which increased with the quantity of Elmiron they had consumed. Per the study, about a quarter of patients with “significant exposure” to the drug—a prescription of more than 500 grams taken for an average of 15 years— had developed signs of eye damage and vision loss, most often pigmentary maculopathy, macular degeneration, or retinal maculopathy. These patients were often misdiagnosed with other retinal conditions, including age-related macular degeneration and pattern dystrophy.
The researchers noted that Elmiron has been a broadly prescribed treatment for IC for decades and that hundreds of thousands of people had likely been exposed to the “vision-threatening” drug. They also recommended that patients using Elmiron go in for additional screenings once a year or more to keep tabs on potential vision loss.
Dr. Vora told the American Academy of Ophthalmology, “It’s unfortunate. You have a patient with a chronic condition like interstitial cystitis, for which there is no cure and no effective treatment. They get put on these medications because it’s thought to have few side effects and few risks, and no one thinks about it again. And year after year, the number of pills they’re taking goes up and up.”
November 2019 – Emory large cohort study
The 2019 report from the Elmiron Eye Center was the first national multicenter study to demonstrate a link between Elmiron usage and vision loss. Researchers Nieraj Jain, Alexa L. Li, Yinxi Yu, and Brian L. VanderBeek examined data gathered between 2002 and 2016 by a large US medical claims database. They compared more than 4,500 users of the drug Elmiron with more than 23,000 control patients over periods of five and seven years.
At the five-year mark, they found no significant link between Elmiron use and eye injury. However, at seven years, they found that patients using Elmiron had much higher odds of a diagnosis of atypical macular degeneration linked to vision impairment.
November 2019 – Harvard case report
A November 2019 case study from Rachel M. Huckfeldt and Demetrios G. Vavvas at the Harvard Medical School Department of Ophthalmology provided the first evidence that retina damage and vision loss caused by Elmiron could continue and significantly worsen even after the patient stopped using the product.
The study focused on a female patient who first came to the clinic with complaints of blurred vision and difficulty seeing at night after using Elmiron for 18 years. The patient stopped using Elmiron one year after visiting, but on examinations six and eight years later, clinicians found significantly more severe Elmiron-related eye damage and vision impairment. Tests showed that she had no genetic predisposition for eye disease.
The researchers wrote, “The present case adds a new layer of concern by demonstrating progressive maculopathy continuing for up to 6 years after the cessation of PPS. The case emphasizes the need for a screening regiment that balances the demands of patients and physicians with the importance of prompt identification of early toxicity.”
April 2020 – UCLA study
A cross-sectional study published in early 2020 by the Stein Eye Institute at the University of California, Los Angeles supported the findings of earlier researchers, determining that nearly a quarter of patients studied had macular degeneration as a result of PPS exposure.
Researchers Derrick Wang, Adrian Au, Frederic Gunnemann, Assaf Hilely, Jackson Scharf, Khoi Tran, Michel Sun, Ja-Hong Kim, and David Sarraf also provided the first evidence that more significant exposure to Elmiron led to more significant atrophy of the retina. Specifically, they found that “patients exposed to greater than 1500 g of PPS are at significant risk of retinal toxicity.”
June 2020 – Multicenter study
The multicenter study from the Emory Eye Center, the Casey Eye Institute, and the Kellogg Eye Center confirmed the results found in the 2019 Harvard case report: that damage to the eye can continue after Elmiron use is stopped. In a retrospective chart review, researchers Rachel Shah, Riley Lyons, Joseph Simonett, Mark E. Pennesi, Rajesh C. Rao, and Nieraj Jain identified twelve female subjects who had been diagnosed with pigmentary maculopathy and had stopped using Elmiron.
Of the twelve individuals, ten reported worsening of visual symptoms after discontinuing PPS. The researchers wrote, “PPS maculopathy continues to evolve even after drug cessation. Areas of RPE atrophy continue to grow, coalesce, and encroach on the foveal center. This may pose a long-term threat to central vision, even remote from the time of medication use. Affected patients should be counseled appropriately, and screening programs should be instituted to promote early detection.”
Who are the defendants in the Elmiron eye damage lawsuit?
The primary defendant in the Elmiron lawsuits is Janssen Pharmaceuticals. The pharmaceutical arm of Johnson & Johnson, their website states their work involves “fighting sickness with science, improving access with ingenuity, and healing hopelessness with heart.”
Founded in 1953 in Belgium, the company was acquired by Johnson & Johnson in 1961 and is now part of Johnson & Johnson Pharmaceutical Research and Development. They focus specifically on areas of medicine including cardiovascular health & metabolism, immunology, infectious diseases & vaccines, neuroscience, oncology, and pulmonary hypertension.
According to their annual report, in 2022 Janssen’s parent company, Johnson & Johnson, reported adjusted net earnings of $27 billion. They are currently undergoing a number of litigations beyond the Elmiron eye damage litigation, including a suit linking Johnson & Johnson talcum powder to ovarian cancer.
Despite the growing number of Elmiron eye damage lawsuits, Janssen Pharmaceuticals is still marketing Elmiron as an effective treatment with only minor side effects.
Are doctors still prescribing Elmiron?
Elmiron is still on the market and is still being prescribed to patients. In 2020, the FDA required a change in Elmiron packaging and the label was updated to include a warning of retinal pigmentary changes. No Elmiron recall has been made to date.
Plaintiffs in the Elmiron lawsuits claim that Janssen Pharmaceuticals was aware of the potential harm and failed to disclose the risk until recently. Under U.S. law, consumers are entitled to information about the potential risks of a product before they choose to use it. That is the basis of the manufacturer’s “duty to warn,” and the case the lawyers at Wallace Miller are making in the Elmiron lawsuit: that the defendants should have better informed their patients about the potential for harm, including vision loss.
Ultimately, it is up to consumers to decide what risks they are willing to take. As a result, there is no planned Elmiron recall, as consumers may decide that the advantages of the medication are worth the risks of potentially permanent vision damage. However, if patients would have made a different choice had they been fully informed, they should be compensated for the physical, emotional, and financial damages caused by the drug. The Elmiron vision damage lawsuits hope to secure those damages for the plaintiffs involved.
How do you know if Elmiron damaged your vision?

Digital retinal photography of an eyeball. Photograph by Pixabay.
The long-term use of Elmiron has been linked to a number of diagnoses, including pigmentary maculopathy, retinal maculopathy, and macular degeneration. Vision impairment due to Elmiron can also be misdiagnosed as an age-related macular degeneration.
Common signs of pigmentary maculopathy include blurred or distorted vision, trouble adjusting to dark or dim light, vision loss, straight lines appearing wavy or curvy, and difficulty reading.
Even if you’re not experiencing symptoms yet, you could be affected if you’re taking Elmiron. See a retinal specialist as soon as possible and ask about the possibility of pigmentary maculopathy.
Who qualifies for Elmiron lawsuits?
Are you concerned you may be suffering vision loss as a result of using Elmiron? You may be eligible to participate in the litigation if you meet the following Elmiron lawsuit criteria:
You used the prescription drug Elmiron on a daily basis for at least one year. The typical dosage is 100 mg, three times a day, as prescribed by a gynecologist or urologist for treatment of interstitial cystitis.
You have a form of retinal degradation or eye damage. These conditions may include pigmentary maculopathy, pigmentation macular degeneration, retinal pigmentary epithelium (RPE), macular degeneration, and age-related macular degeneration (AMD).
What if I don’t have a diagnosis?
You may still be eligible for the Elmiron lawsuit if you have certain symptoms of vision loss and are willing to see a doctor in the next few months. These symptoms include:
- Blurred vision
- Distorted vision
- Difficulty reading
- Colors appear dull or less vivid
- Dimming vision
- Central vision loss
- Trouble adjusting to dim light
- Spots in your field of vision
- Straight lines appear curvy or wavy
- Night vision problems
- Blindness
- Eye pain
If you believe you have been harmed by taking Elmiron, reach out to Wallace Miller at 312-261-6193 or fill out our online questionnaire.
Why should I file an Elmiron lawsuit?
Plaintiffs in the Elmiron lawsuits are claiming that the manufacturer was aware of the drug’s potential to cause vision damage and failed to disclose that risk until recently.
As this is a personal injury claim, patients who have been prescribed Elmiron may be eligible for compensation for injuries caused by the drug, including medical bills, loss of wages, pain and suffering, and emotional distress. If Janssen Pharmaceuticals is found to be grossly negligent (i.e., if they acted with reckless disregard for the safety or lives of others, consciously violating others’ rights) or acting maliciously (i.e., they consciously and intentionally acted to harm) the Elmiron lawsuit settlements may include punitive damages as well. These amounts exceed simple compensation for the damages sustained and are awarded to punish the defendant.
Is this an Elmiron class action lawsuit?
Hundreds of Elmiron users in the US have filed claims against Janssen Pharmaceuticals. However, because the specific medical circumstances differ from plaintiff to plaintiff, these cases are mass torts, rather than class actions.
Class actions and mass torts are similar in that they enable the courts to resolve many claims at once via a consolidation process. But in order for cases to be consolidated in a class action, certain requirements must be satisfied, including a common issue or injury shared by all plaintiffs, and the eligibility of one case to act as a representative of the whole class.
Mass tort cases, such as those in the Elmiron MDL, are all filed and awarded independently. This is almost always the case for lawsuits that deal with physical (rather than economic) injuries, because the medical circumstances of every plaintiff will be unique. Since the damages in the Elmiron vision loss lawsuit differ from person to person but are all being brought against Janssen Pharmaceuticals, the mass tort claims have been consolidated into a multidistrict litigation, or MDL.
If a global settlement is reached in the Elmiron MDL, the individual lawsuits will be divided into tiers to determine the settlement awards, allowing the process to move more quickly. As a result, if a settlement is eventually reached, each plaintiff involved in an Elmiron mass tort will receive compensation for their individual circumstances.
If the case settles, how much can I receive from an Elmiron lawsuit settlement?
In any litigation, there is no guarantee of a settlement until the award is confirmed. However, if the case does end in a settlement, there are a number of factors that may influence the amounts. The bellwether trials scheduled for later this year will help our Elmiron lawyers determine what a typical settlement amount could be. However, as the litigation is still in its early stages and a settlement is not guaranteed, it’s difficult to estimate the average Elmiron settlement amounts at this point.
Since this is a mass tort and not a class action, if a settlement is reached, the amount each plaintiff receives will depend on their situation and the extent of their injuries. For example, a plaintiff in the Elmiron lawsuit who has completely lost their vision could receive more than a plaintiff who suffers from increased sensitivity to light. For efficiency’s sake, if a resolution occurs, the Elmiron settlements will likely be divided up into tiers of users who receive set amounts.
Our Elmiron lawyers are committed to fighting for the best possible outcome for all plaintiffs involved. Your lawyer will work with you throughout the process to keep you informed on updates in the case, including the likelihood of a settlement and the potential award amounts.
When will I find out about a possible Elmiron settlement?
Judge Brian Martinotti has called for bellwether trials that are scheduled to take place in the first half of 2023. Although it is always difficult to predict the course of a litigation, these trials will help the attorneys determine whether an Elmiron settlement is likely or whether the case will go to trial. To date, no trials have taken place.
Mass tort cases are complex, and the Elmiron MDL is no exception. Cases like these tend to take three to five years from initial filings to resolution. Your Elmiron lawyer will keep in contact with you to let you know any progress in the case.
What is a bellwether trial?
A bellwether trial is essentially a test trial designed to get a sense of how other cases in a large litigation will go. It’s named after an old practice of tying a bell to the neck of a lead sheep in a flock in order to monitor the whole flock’s movements, and similarly, it can help the attorneys determine which way a litigation is heading.
When there is a large pool of lawsuits against one party, as in the Elmiron MDL, a case that is representative of the group at large is selected as the bellwether trial. Their existing Elmiron lawsuit is brought to trial early by their attorneys, and the decision of the court helps everyone involved in the process foresee how future litigations will go. These cases don’t guarantee any of the other case outcomes, but provide a representative sample for future Elmiron vision loss lawsuits.
For defendants, the outcome of a bellwether trial can help them assess whether they want to pursue further litigation or settle out of court. For plaintiffs, it’s an opportunity to see how effective their evidence is and determine what strategies will be most successful going forward. It can also be useful for judges and legislators because it allows them to get a sense of trends in the industry as a whole.
If the upcoming bellwether trials favor the plaintiffs in the Elmiron lawsuits, it’s likely, though not certain, that the defendants will offer a global settlement. If this happens, the process will begin to move more quickly and Elmiron victims may receive a settlement sooner.
What happens if my Elmiron lawsuit doesn’t settle?
The Elmiron lawyers at Wallace Miller believe that this is a strong case and that the defendants will be in a position to negotiate a settlement. However, there is always the chance that they will reject a global settlement and cases will go to trial. In that situation, each individual Elmiron lawsuit will be decided in a jury trial. Your Elmiron attorneys will provide information and guidance at every step of the way.
How much will my Elmiron lawsuit cost?
It may surprise you to hear this, but you will not incur any kind of fee from Wallace Miller unless we win your Elmiron lawsuit case. Wallace Miller relies entirely on successful settlements to pay operation fees, so there is no out-of-pocket cost for our clients.
Can I still file an Elmiron lawsuit?
It is likely that plaintiffs will be able to continue to bring new Elmiron cases in the months and years ahead. Even if a settlement is reached in 2023, the courts will probably set it up in order to take into account those filing claims going forward.
If you think you may have been harmed by taking Elmiron, get in touch with Wallace Miller today at 312-261-6193 or fill out our online questionnaire to find out if you can file an Elmiron case.
What information do I need for an Elmiron lawsuit?
Evidence is crucial when a plaintiff is bringing a case against a manufacturer. When you discuss your Elmiron eye damage lawsuit with us, we will ask about your history of Elmiron use as well as your diagnoses.
In order to file an Elmiron lawsuit, you will need a diagnosis of an eligible condition from a qualified medical professional. However, if you have symptoms of retinal degeneration, you may be able to speak to your doctor and qualify for the case if you are diagnosed.
Based on the information you provide, we will assess whether we think you have a case against the defendants for Elmiron-related eye damage. Ultimately, the Elmiron lawyers at Wallace Miller are responsible for using the evidence available to argue your case effectively.
Wallace Miller: Investigating claims about Elmiron vision loss
At Wallace Miller, we believe in two things: relationships and results. Whenever people are seriously injured or killed by the negligence of others, there’s a story to tell. Our law firm builds relationships with our clients, making sure to collect information that is tailored to each case so that we can properly tell your story in order to produce results.

Left to right: Nicholas P. Kelly, Edward A. Wallace, Molly Condon Wells, Mark R. Miller, Jessica Wieczorkiewicz, Timothy E. Jackson.
Wallace Miller primarily focuses on protecting the rights of victims of negligence and fraud. We are routinely appointed by federal and state courts to serve as leaders in national litigation, and our attorneys have obtained ground-breaking verdicts in a variety of cases across the nation. But more importantly, we are committed to obtaining justice for each and every one of our clients.
We know that there are many choices available to you when looking for a lawyer to represent you. What sets us apart from others is our commitment to our clients, our ability to handle complex high-stakes litigation, and our outstanding track record of success.
We only take on cases that the firm has faith in and believe can make a difference in our clients’ lives, including the Elmiron lawsuit.
Start your Elmiron lawsuit — Contact Wallace Miller now
If you have been harmed by taking Elmiron, reach out to Wallace Miller at 312-261-6193 to discuss your potential vision loss lawsuit or fill out our online questionnaire for a case assessment in minutes.

